Pfizer PFE announced additional data from the phase II/III EPIC-SR study, which evaluated the use of its COVID-19 treatment pill Paxlovid in patients who are at standard risk for developing severe COVID-19 infection.
As was reported last year, the EPIC-SR study had failed to achieve its primary endpoint of self-reported, sustained alleviation of all symptoms for four consecutive days. An updated analysis of the data also showed that the study achieved a non-significant 51% relative reduction in the key secondary endpoint of hospitalization or death in standard risk population.
A sub-group analysis also revelated a non-significant 57% relative reduction in hospitalization or death in vaccinated adults having at least one risk factor for progression to severe COVID-19 who were treated with Paxlovid. Additional analyses also showed that treatment with Paxlovid resulted in a nominally significant 62% decline in COVID-related medical visits per day across all patients compared to treatment with placebo.
Following the low rate of hospitalization or death in the standard-risk population, Pfizer decided to stop enrolling patients in the EPIC-SR study. PFE will instead focus on using the drug as a treatment for patients who are at high-risk of developing severe COVID infection.
Though the data from the EPIC-SR study is not all statistically significant, Pfizer intends to submit the same along with the new drug application (NDA), which will seek the FDA’s approval to treat appropriate individuals who are at high risk of progression to severe illness. The NDA will be based on data from the phase II/III EPIC-HR study, which evaluated Paxlovid in high-risk patients for developing severe COVID-19 infection.
In the year so far, shares of Pfizer have declined 18.9% compared with the industry’s 2.7% fall.
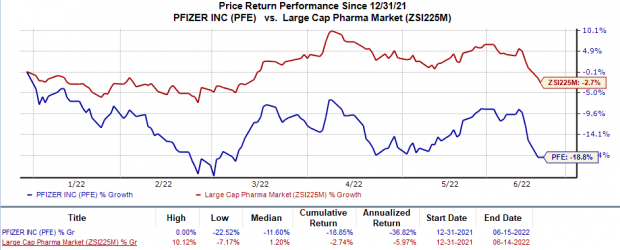
Image Source: Zacks Investment Research
Presently, Paxlovid is authorized for emergency use by the FDA to treat mild-to-moderate COVID-19 in adult and pediatric patients (12 years and older and weighing at least 40 kg) who are at an increased risk of hospitalizations or death.
During the first quarter of 2022, Pfizer generated $1.47 billion from Paxlovid. For the full year, PFE expects to generate revenues of $22 billion from Paxlovid sales. It also confirmed that the above-mentioned data will not impact its revenue guidance for full year 2022.
Like Pfizer, another pharma giant, which is also marketing its own COVID-19 treatment pill is Merck MRK. Similar to Paxlovid, the FDA granted emergency use authorization to MRK’s Lagevrio (molnupiravir) last December to treat high-risk adults with mild-to-moderate COVID-19.
Merck’s Lagevrio generated sales of $3.25 billion during the first quarter of 2022. MRK expects sales in the range of $5-$5.5 billion from Lagevrio in 2022.
Apart from Lagevrio and Paxlovid, multiple companies are marketing their own therapies for treating COVID-19. One such therapy is Veklury (remdesivir), marketed by Gilead Sciences GILD, which has been approved in the United States since 2020. Gilead’s Veklury is approved by the FDA to treat moderate-to-severe COVID-19 in adults and pediatric patients who are at high risk of progression to severe COVID-19. In first-quarter 2022, Gilead recorded $1.5 billion from Veklury sales.
However, unlike Gilead’s Veklury, which can only be administered in hospitals, Pfizer’s Paxlovid and Merck’s Lagevrio can be prescribed for at-home treatments.
Pfizer Inc. Price

Pfizer Inc. price | Pfizer Inc. Quote
Zacks Rank & Stocks to Consider
Pfizer currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the overall healthcare sector is Sesen Bio SESN, which carries a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Sesen Bio’s loss per share estimates for 2022 has declined from 33 cents to 32 cents in the past 60 days. Shares of SESN have fallen 24.2% in the year-to-date period.
Earnings of Sesen Bio beat estimates in three of the last four quarters and missed the mark on one occasion, the average surprise being 69.9%. In the last reported quarter, Sesen Bio delivered an earnings surprise of 100%.
Zacks' Top Picks to Cash in on Electric Vehicles
Big money has already been made in the Electric Vehicle (EV) industry. But, the EV revolution has not hit full throttle yet. There is a lot of money to be made as the next push for future technologies ramps up. Zacks’ Special Report reveals 5 picks investors
See 5 EV Stocks With Extreme Upside Potential >>Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
SESEN BIO, INC. (SESN): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.


