Denmark-based biopharmaceutical company, Orphazyme A/S ORPH announced an update on the timeline of its planned resubmission of the new drug application (“NDA”) for its investigational product candidate, arimoclomol, for the treatment of Niemann-Pick disease type C (“NPC”), a rare genetic disorder. Currently, there are no approved treatments for the given indication in the United States.
The company is planning to request a Type C Meeting with the FDA in the second quarter of 2022. Subject to discussions with the regulatory body, Orphazyme plans to resubmit the NDA for arimoclomol in the second half of 2022.
Shares of Orphazyme were up 8.3% on Friday following the announcement of the news. However, the stock has plunged 82.1% in the past year compared with the industry’s decrease of 40.4%.
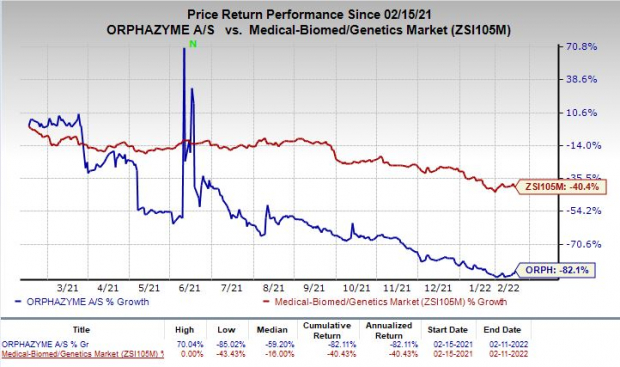 Image Source: Zacks Investment Research
Image Source: Zacks Investment Research
In June 2021, the FDA issued a Complete Response Letter (“CRL”) to the NDA for arimoclomol for the treatment of NPC. The company had a Type A Meeting with the FDA in October 2021, wherein the regulatory body requested additional data, information and analyses on certain topics of the CRL.
In line with recommendations from the regulatory body during the Type A Meeting, the company is looking to request a Type C Meeting to discuss the additional data, information and analyses related to certain topics mentioned in the CRL.
In November 2020, Orphazyme filed a marketing authorization application seeking approval for arimoclomol for the treatment NPC to the European Medicines Agency ("EMA"). An opinion from the EMA’s Committee for Medicinal Products for Human Use is expected later in the first quarter of 2022.
The FDA has already granted Fast-Track Designation, Breakthrough Therapy Designation and Rare Pediatric Disease Designation to arimoclomol for treating NPC. The candidate has received Orphan Drug Designation for addressing NPC both in the United States and Europe.
Successful development of arimoclomol remains a primary focus for the company.
Zacks Rank & Stocks to Consider
Orphazyme currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector include Axsome Therapeutics, Inc. AXSM, Editas Medicine, Inc. EDIT and Kaleido Biosciences, Inc. KLDO, all sporting a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Axsome Therapeutics’ loss per share estimates have narrowed 0.8% for 2022 over the past 60 days.
Earnings of Axsome Therapeutics have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion.
Editas Medicine’s loss per share estimates have narrowed 1.4% for 2022 over the past 60 days.
Earnings of Editas Medicine have surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions.
Kaleido Biosciences’ loss per share estimates have narrowed 11.3% for 2022 over the past 60 days.
Earnings of Kaleido Biosciences have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion.
Just Released: Zacks Top 10 Stocks for 2022
In addition to the investment ideas discussed above, would you like to know about our 10 top buy-and-hold tickers for the entirety of 2022?
Last year's 2021 Zacks Top 10 Stocks portfolio returned gains as high as +147.7%. Now a brand-new portfolio has been handpicked from over 4,000 companies covered by the Zacks Rank. Don’t miss your chance to get in on these long-term buys
Access Zacks Top 10 Stocks for 2022 today >>Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Axsome Therapeutics, Inc. (AXSM): Free Stock Analysis Report
Editas Medicine, Inc. (EDIT): Free Stock Analysis Report
Kaleido Biosciences, Inc. (KLDO): Free Stock Analysis Report
Orphazyme AS Sponsored ADR (ORPH): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.


