Novavax NVAX announced positive data from the pediatric expansion of the phase III PREVENT study, which evaluated its protein-based COVID-19 vaccine, NVX-CoV2373, in adolescents aged 12-17 years.
The pediatric expansion study achieved its primary effectiveness endpoint of NVX-CoV2373 generating neutralizing antibodies in adolescents, similar to the antibody responses in young adult patients (aged between 18 and 26 years) who were administered the vaccine in the phase III PREVENT study. In fact, the antibody responses were 1.5 fold higher in adolescents than young adults.
Please note that the results of this study are based on the data accrued last year between May 24, 2021 and Sep 27, 2021, the period during which the Delta variant was the dominating strain in the United States. NVX-CoV2373 demonstrated an efficacy of 82% against the Delta variant.
Following a two-dose vaccine regimen in adolescents, IgG responses against spike proteins of several variants including Alpha, Beta, Gamma and Omicron were 2-3 fold higher than those observed in adults. Further, the functional immune response in adolescents was 2.4-4 fold higher than in adults against these evaluated variants.
Based on preliminary safety data, Novavax’s COVID vaccine was well-tolerated among the study participants. The company expects to use data from this study to seek approval for the use of its vaccine in adolescents and will file the same with multiple health regulators worldwide during first-quarter 2022.
NVAX also plans to start additional studies during second-quarter 2022, which will evaluate NVX-CoV2373 in younger age groups.
Shares of Novavax have plunged 69.6% in the trailing 12 months in comparison with the industry’s 39.9% decline.
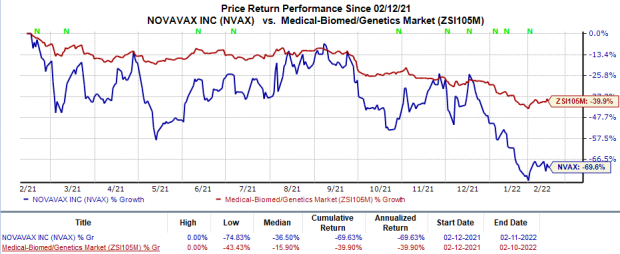 Image Source: Zacks Investment Research
Image Source: Zacks Investment Research
We remind investors that Novavax is yet to receive approval for its COVID vaccine in the United States. Last month, the company filed an Emergency Use Authorization with the FDA for NVX-CoV2373 in adults.
NVX-CoV2373 has already received approval for emergency use in adults in highly populated markets like Australia, Europe, India and Indonesia. The company has also submitted regulatory filings seeking approval for NVX-CoV2373 in multiple markets like Canada, Japan and UAE, which are currently under review.
If approved, Novavax’s protein vaccine will face stiff competition from the COVID vaccines developed by Moderna MRNA and Pfizer PFE/BioNTech BNTX, which currently dominate the U.S. market. In fact, the booster doses of these vaccines are also approved for use in adults in the United States.
We note that the vaccines developed by Moderna and Pfizer/BioNTech are based on the mRNA technology and require a two-dose primary regimen. In fact, the COVID vaccines developed by Pfizer/BioNTechand Moderna are currently the only ones that have received full approval in the United States.
We note that Pfizer/BioNTEch’s COVID vaccine is already approved by the FDA for emergency use in individuals aged five years and above. Meanwhile, Moderna has filed for emergency use of its COVID vaccine in adolescents aged between 12 and 17 years of age, which is currently under review by the FDA.
Novavax, Inc. Price

Novavax, Inc. price | Novavax, Inc. Quote
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Infrastructure Stock Boom to Sweep America
A massive push to rebuild the crumbling U.S. infrastructure will soon be underway. It’s bipartisan, urgent, and inevitable. Trillions will be spent. Fortunes will be made.
The only question is “Will you get into the right stocks early when their growth potential is greatest?”
Zacks has released a Special Report to help you do just that, and today it’s free. Discover 5 special companies that look to gain the most from construction and repair to roads, bridges, and buildings, plus cargo hauling and energy transformation on an almost unimaginable scale.
Download FREE: How to Profit from Trillions on Spending for Infrastructure >>Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE): Free Stock Analysis Report
Moderna, Inc. (MRNA): Free Stock Analysis Report
Novavax, Inc. (NVAX): Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.


