Abbott ABT recently received FDA approval for its pioneering AVEIR dual chamber (DR) leadless pacemaker system. The approval comes following the recent late-breaking clinical trial data showing that the system is safe and effective for treating abnormal heart rhythms.
The latest development significantly increases access to leadless pacing for millions of people across the United States.
Significance of AVEIR DR Devices
The AVEIR DR leadless pacing system is made up of two devices — the previously approved AVEIR VR single-chamber device, which paces the right ventricle, and the recently approved AVEIR AR single-chamber device, which paces the right atrium. Smaller than the AAA battery, each AVEIR pacemaker is implanted via a minimally invasive procedure.
AVEIR DR devices provide synchronized or coordinated cardiac pacing between two leadless pacemakers based on the person's clinical needs using Abbott's novel i2i (implant-to-implant) technology. The breakthrough technology utilizes high-frequency pulses to relay messages via the naturally conductive characteristics of the body's blood between each leadless pacemaker.
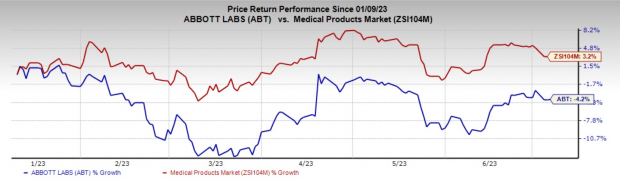
Image Source: Zacks Investment Research
Each implant communicates beat-to-beat with a paired, co-implanted device to support dual-chamber therapy. This conductive communication is critical because it uses far less battery current than inductive, radio frequency or Bluetooth communication.
AVEIR leadless pacemakers are designed for retrieval, which will help if therapy needs to be changed or the device needs to be replaced in the future.
News in Detail
With more than 80% of people requiring pacing in both the right atrium and the right ventricle, the currently available leadless pacing options have been limited to single-chamber ventricular devices. This stems from the significant engineering challenge of seamless, wireless synchronization of two leadless pacemakers.
The AVEIR DR system, which is roughly one-tenth the size of a traditional pacemaker, is also designed to provide real-time pacing analysis. This enables physicians to assess the proper placement of the device during the procedure and before implanting the device inside the heart chamber.
Industry Prospects
Per a Research report, the global leadless cardiac pacemaker market is expected to witness a CAGR of 17.2% by 2028.
Recent Developments
In June, Abbott announced a collaboration with the American Diabetes Association, to gain a deeper understanding of the role of Continuous Glucose Monitoring systems in personalized therapeutic nutrition for people with diabetes.
During the same month, ABT’s FreeStyle Libre 2 system became the first and only CGM system to be nationally reimbursed in France for people who use basal insulin.
Price Performance
In the past six months, Abbott shares have decreased 4.2% against the industry’s rise of 3.2%.
Zacks Rank and Other Key Picks
Abbott currently carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks in the overall healthcare sector are Haemonetics HAE, Zimmer Biomet ZBH and SiBone SIBN. While Haemonetics sports a Zacks Rank #1 (Strong Buy), Zimmer Biomet and SiBone each carry a Zacks Rank #2. You can see the complete list of today’s Zacks #1 Rank stocks here.
Haemonetics’ stock has risen 25.6% in the past year. The Zacks Consensus Estimate for Haemonetics’ earnings per share (EPS) has increased from $3.55 to $3.56 for fiscal 2024 and remained constant at $3.96 for fiscal 2025 in the past 30 days.
HAE’s earnings beat estimates in each of the trailing four quarters, the average surprise being 12.21%. In the last reported quarter, the company registered an earnings surprise of 13.24%.
The Zacks Consensus Estimate for Zimmer Biomet’s 2023 EPS has remained constant at $7.45 in the past 30 days. Shares of the company have improved 34.2% in the past year against the industry’s 21.6% decline.
ZBH’s earnings beat estimates in each of the trailing four quarters, the average surprise being 7.38%. In the last reported quarter, the company recorded an earnings surprise of 13.86%.
The Zacks Consensus Estimate for SiBone’s 2023 loss per share has narrowed from $1.44 to $1.42 in the past 30 days. SIBN shares have improved 92.3% in the past year compared with the industry’s 9% growth.
SIBN’s earnings beat estimates in three of the trailing four quarters and missed the same in one, the average surprise being 11.11%. In the last reported quarter, the company recorded an earnings surprise of 21.95%.
7 Best Stocks for the Next 30 Days
Just released: Experts distill 7 elite stocks from the current list of 220 Zacks Rank #1 Strong Buys. They deem these tickers "Most Likely for Early Price Pops."
Since 1988, the full list has beaten the market more than 2X over with an average gain of +24.2% per year. So be sure to give these hand-picked 7 your immediate attention.
See them now >>Abbott Laboratories (ABT) : Free Stock Analysis Report
Haemonetics Corporation (HAE) : Free Stock Analysis Report
Zimmer Biomet Holdings, Inc. (ZBH) : Free Stock Analysis Report
SiBone (SIBN) : Free Stock Analysis Report
To read this article on Zacks.com click here.
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.


